- Deuterium oxide MSDS
- ?Is drinking deuterium oxide harmful
- Core invasion studies using deuterium oxide tracer
- Pharmacological use of deuterium oxide
- Deuterium oxide vs. deuterium depleted
- NMR spectroscopy using Deuterium oxide
- Treatment of semiconductor devices using deuterium oxide
- Manufacturing of duterium lamp using deuterium oxide
- How to create deuterium oxide
- Deuterium oxide application: Fiber Optics
آخرین مطالب
امکانات وب
 SAFETY DATA SHEET
according
to the Global Harmonized System
Version:
GHS 1
1. Identification of the
substance/mixture and of the company/undertaking
DEUTERIUM OXIDE Product name 1.004.025, 1.004.050 1.031.050 1.032.050 1.033.025, 1.033.050 (Cat. No.(s Laboratory chemicals, Manufacture of substances Identified uses Mesbah Energy Co Arak Science & Technology Park Shahid Ghoddousi Blvd., Arak, Iran Tel-Fax: +98 86 32246912 Email: [email protected] company/undertaking Identification
2. Hazards identification
No data available Classification of the substance or mixture No data available Label elements No data available Other hazards
3. Composition/information on ingredients
Heavy water, Water-d2 Substances synonyms D2O Formula 7789-20-0 .CAS NO
4. First aid measures
Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of dangerous area General If breathed in, move person to fresh air. If not breathing, give artificial respiration. Consult a physician Inhalation Wash with soap and plenty of water. Consult a physician Skin contact Flush eyes with water as a precaution History of deuterium oxide...
SAFETY DATA SHEET
according
to the Global Harmonized System
Version:
GHS 1
1. Identification of the
substance/mixture and of the company/undertaking
DEUTERIUM OXIDE Product name 1.004.025, 1.004.050 1.031.050 1.032.050 1.033.025, 1.033.050 (Cat. No.(s Laboratory chemicals, Manufacture of substances Identified uses Mesbah Energy Co Arak Science & Technology Park Shahid Ghoddousi Blvd., Arak, Iran Tel-Fax: +98 86 32246912 Email: [email protected] company/undertaking Identification
2. Hazards identification
No data available Classification of the substance or mixture No data available Label elements No data available Other hazards
3. Composition/information on ingredients
Heavy water, Water-d2 Substances synonyms D2O Formula 7789-20-0 .CAS NO
4. First aid measures
Consult a physician. Show this safety data sheet to the doctor in attendance. Move out of dangerous area General If breathed in, move person to fresh air. If not breathing, give artificial respiration. Consult a physician Inhalation Wash with soap and plenty of water. Consult a physician Skin contact Flush eyes with water as a precaution History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 101
 You may have
wondered whether you can you drink heavy water? Is it radioactive? Is it safe?
Deuterium oxide or
heavy water is a form of water that contains a larger than normal amount of the
hydrogen isotope deuterium, rather than the common hydrogen-1 isotope that
makes up most of the hydrogen in normal water. Accordingly, some or most of the
hydrogen atoms in deuterium oxide contain a neutron, causing each hydrogen atom
to be about twice as heavy as a normal hydrogen atom. The increased weight of
the hydrogen in the water thus makes it slightly more dense.
Deuterium oxide is
not radioactive. So, if you drink heavy water, you don't have to stress over
radiation harming. It's not
totally safe to drink, however, because the biochemical reactions in your cells
are affected by the distinction in the mass of the hydrogen atoms and how well
they shape hydrogen bonds.
If you drank a considerable
volume of deuterium oxide, you may feel dizzy because deuterium oxide would
change the density of the liquid in your inner ear.
Deuterium oxide
damages the ability of mitotic spindles cells to repair their DNA and replicate.
If you could replace 25-50% of the regular hydrogen in your History of deuterium oxide...
You may have
wondered whether you can you drink heavy water? Is it radioactive? Is it safe?
Deuterium oxide or
heavy water is a form of water that contains a larger than normal amount of the
hydrogen isotope deuterium, rather than the common hydrogen-1 isotope that
makes up most of the hydrogen in normal water. Accordingly, some or most of the
hydrogen atoms in deuterium oxide contain a neutron, causing each hydrogen atom
to be about twice as heavy as a normal hydrogen atom. The increased weight of
the hydrogen in the water thus makes it slightly more dense.
Deuterium oxide is
not radioactive. So, if you drink heavy water, you don't have to stress over
radiation harming. It's not
totally safe to drink, however, because the biochemical reactions in your cells
are affected by the distinction in the mass of the hydrogen atoms and how well
they shape hydrogen bonds.
If you drank a considerable
volume of deuterium oxide, you may feel dizzy because deuterium oxide would
change the density of the liquid in your inner ear.
Deuterium oxide
damages the ability of mitotic spindles cells to repair their DNA and replicate.
If you could replace 25-50% of the regular hydrogen in your History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 185
 Fig 1.Deuterium oxide tracer can be used
in oil exploration studies
The main objective
of a coring operation is to get accurate information about the oil reservoir. Parameters
like oil and water composition determine the saturation levels of a reservoir.
Information about the reservoir is evaluated by analysis of the collected cores
and samples.
In case of drilling
mud core invasion, the recovered liquid does not represent the native liquid
but rather a mixture of drilling fluid and reservoir fluid. The amount of
coring fluid contamination contained in the formation water of a core sample
can be determined using a tracer.
Deuterium
oxide or heavy water can be used to trace water base mud systems. Prior to
coring a quantity of deuterium oxide is added to the aqueous drilling fluid and
samples of the coring fluid taken periodically before, during and after the coring
process. The levels of deuterium in the extracted water and coring fluid
samples can determine the contamination degree. History of deuterium oxide...
Fig 1.Deuterium oxide tracer can be used
in oil exploration studies
The main objective
of a coring operation is to get accurate information about the oil reservoir. Parameters
like oil and water composition determine the saturation levels of a reservoir.
Information about the reservoir is evaluated by analysis of the collected cores
and samples.
In case of drilling
mud core invasion, the recovered liquid does not represent the native liquid
but rather a mixture of drilling fluid and reservoir fluid. The amount of
coring fluid contamination contained in the formation water of a core sample
can be determined using a tracer.
Deuterium
oxide or heavy water can be used to trace water base mud systems. Prior to
coring a quantity of deuterium oxide is added to the aqueous drilling fluid and
samples of the coring fluid taken periodically before, during and after the coring
process. The levels of deuterium in the extracted water and coring fluid
samples can determine the contamination degree. History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 133
 Fig 1.Development of deuterated drugs
using deuterium oxide
Deuterium oxide or
heavy water is widely used in studies of metabolism of drugs in humans and
other animals. Deuterium from deuterium oxide (D2O) can be exchanged
directly into finished drug compounds.
A deuterium
containing or deuterated drug is a kind of medicine which one or more of its
hydrogen atoms are substituted by deuterium atoms. Metabolic studies have shown
that slower metabolism of deuterium containing drugs often allows for longer
effective benefit, smaller or less frequent doses and fewer side effects of the
drug.
In April 2017 Food
and Drug Administration (FDA) approved the first deuterated drug, AUSTEDO™ by
Teva (previously referred to as SD-809). Austedo was developed for the
treatment of chorea, random involuntary twisting movements, which is associated
with Huntington’s disease.
Source:
http://www.tevapharm.com/news/teva_announces_fda_approval_of_austedo_deutetrabenazine_tablets_for_the_treatment_of_tardive_dyskinesia_in_adults_08_17.aspx History of deuterium oxide...
Fig 1.Development of deuterated drugs
using deuterium oxide
Deuterium oxide or
heavy water is widely used in studies of metabolism of drugs in humans and
other animals. Deuterium from deuterium oxide (D2O) can be exchanged
directly into finished drug compounds.
A deuterium
containing or deuterated drug is a kind of medicine which one or more of its
hydrogen atoms are substituted by deuterium atoms. Metabolic studies have shown
that slower metabolism of deuterium containing drugs often allows for longer
effective benefit, smaller or less frequent doses and fewer side effects of the
drug.
In April 2017 Food
and Drug Administration (FDA) approved the first deuterated drug, AUSTEDO™ by
Teva (previously referred to as SD-809). Austedo was developed for the
treatment of chorea, random involuntary twisting movements, which is associated
with Huntington’s disease.
Source:
http://www.tevapharm.com/news/teva_announces_fda_approval_of_austedo_deutetrabenazine_tablets_for_the_treatment_of_tardive_dyskinesia_in_adults_08_17.aspx History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 162
 Unlike deuterium
oxide (heavy water) which is a form of water that contains a larger than normal
amount of the hydrogen isotope deuterium, Deuterium-depleted water (DDW), also
known as light water, has a lower concentration of deuterium than occurs
naturally. Deuterium a heavier isotope of hydrogen which has, in addition to
its one proton, a neutron, that roughly doubles the mass of the hydrogen atom.
All natural water
contains deuterium. Most water contains about 150 ppm. Water with a
concentration less than 140 ppm is considered deuterium-depleted. Despite the fact
that the consumption of deuterium oxide is harmful, based on clinical studies even
seemingly small reduction in deuterium content can improve a number of health
parameters in human.
Scientists uncovered
that healthy cells react well to reduced amounts of deuterium in water.
However, cancer cells are more sensitive to deuterium depletion. Cancer cells, especially
tumor cells, cannot adapt rapidly resulting in tumor regression without any side
effects on healthy cells. They also studied DDW for metabolic disorders - especially
diabetes - with desirable results.
Source:
http://www.dancingwithwater. History of deuterium oxide...
Unlike deuterium
oxide (heavy water) which is a form of water that contains a larger than normal
amount of the hydrogen isotope deuterium, Deuterium-depleted water (DDW), also
known as light water, has a lower concentration of deuterium than occurs
naturally. Deuterium a heavier isotope of hydrogen which has, in addition to
its one proton, a neutron, that roughly doubles the mass of the hydrogen atom.
All natural water
contains deuterium. Most water contains about 150 ppm. Water with a
concentration less than 140 ppm is considered deuterium-depleted. Despite the fact
that the consumption of deuterium oxide is harmful, based on clinical studies even
seemingly small reduction in deuterium content can improve a number of health
parameters in human.
Scientists uncovered
that healthy cells react well to reduced amounts of deuterium in water.
However, cancer cells are more sensitive to deuterium depletion. Cancer cells, especially
tumor cells, cannot adapt rapidly resulting in tumor regression without any side
effects on healthy cells. They also studied DDW for metabolic disorders - especially
diabetes - with desirable results.
Source:
http://www.dancingwithwater. History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 136
 Fig 1. NMR
spectroscopy using Deuterium oxide
The NMR phenomenon is
based on the interaction of the nuclei of specific atomic isotopes with a
static magnetic field. The utility of NMR arise from the fact that chemically
distinct nuclei differ in resonance frequency in the same magnetic field. This
phenomenon is known as the chemical shift.
NMR spectroscopy is
an essential tool for the determination of molecular structure, the study of
molecular dynamics, and the characterization of materials at the molecular
level by chemists, physicists, and molecular biologists.
Deuterium oxide (D2O)
is used in nuclear magnetic resonance spectroscopy when using water as solvent
if the nuclide of interest is hydrogen. This is because the signal from light water
solvent molecules interferes with observing the signal from the molecule of
interest dissolved in it.
Source:
http://chemnmr.colorado.edu/moreinfo/whatisnmr.html
https://en.wikipedia.org/wiki/Heavy_water History of deuterium oxide...
Fig 1. NMR
spectroscopy using Deuterium oxide
The NMR phenomenon is
based on the interaction of the nuclei of specific atomic isotopes with a
static magnetic field. The utility of NMR arise from the fact that chemically
distinct nuclei differ in resonance frequency in the same magnetic field. This
phenomenon is known as the chemical shift.
NMR spectroscopy is
an essential tool for the determination of molecular structure, the study of
molecular dynamics, and the characterization of materials at the molecular
level by chemists, physicists, and molecular biologists.
Deuterium oxide (D2O)
is used in nuclear magnetic resonance spectroscopy when using water as solvent
if the nuclide of interest is hydrogen. This is because the signal from light water
solvent molecules interferes with observing the signal from the molecule of
interest dissolved in it.
Source:
http://chemnmr.colorado.edu/moreinfo/whatisnmr.html
https://en.wikipedia.org/wiki/Heavy_water History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 140
برچسب : نویسنده : 4deuteriumoxide3 بازدید : 123
 Fig 1.Deuterium oxide application:
Deuterium lamp manufacturing
Deuterium lamp (or deuterium
arc lamp) is a low-pressure gas-discharge light source often used in
spectroscopy when constant and intense ultraviolet light is required.
These light sources
utilize a heated tungsten filament that manufactures an arc to the anode in
order to energize molecular deuterium for visible and infrared light
production.
Arc lamps are remarkable
for their high performance in the ultraviolet, with relatively little output in
the visible and infrared. Arc lamps made with ordinary light-hydrogen provide a
very similar UV spectrum to deuterium, and have been utilized as a part of UV
spectroscopes. However, lamps using deuterium have a longer life span and intensity
at the far end of their UV range which is three to five times that of a
standard hydrogen arc bulb, at the similar temperature. Therefore, Deuterium lamps
are considered a superior light source than light-hydrogen arc lamps, for the
shortwave UV range.
Source:
https://en.wikipedia.org/wiki/Deuterium_arc_lamp History of deuterium oxide...
Fig 1.Deuterium oxide application:
Deuterium lamp manufacturing
Deuterium lamp (or deuterium
arc lamp) is a low-pressure gas-discharge light source often used in
spectroscopy when constant and intense ultraviolet light is required.
These light sources
utilize a heated tungsten filament that manufactures an arc to the anode in
order to energize molecular deuterium for visible and infrared light
production.
Arc lamps are remarkable
for their high performance in the ultraviolet, with relatively little output in
the visible and infrared. Arc lamps made with ordinary light-hydrogen provide a
very similar UV spectrum to deuterium, and have been utilized as a part of UV
spectroscopes. However, lamps using deuterium have a longer life span and intensity
at the far end of their UV range which is three to five times that of a
standard hydrogen arc bulb, at the similar temperature. Therefore, Deuterium lamps
are considered a superior light source than light-hydrogen arc lamps, for the
shortwave UV range.
Source:
https://en.wikipedia.org/wiki/Deuterium_arc_lamp History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 157
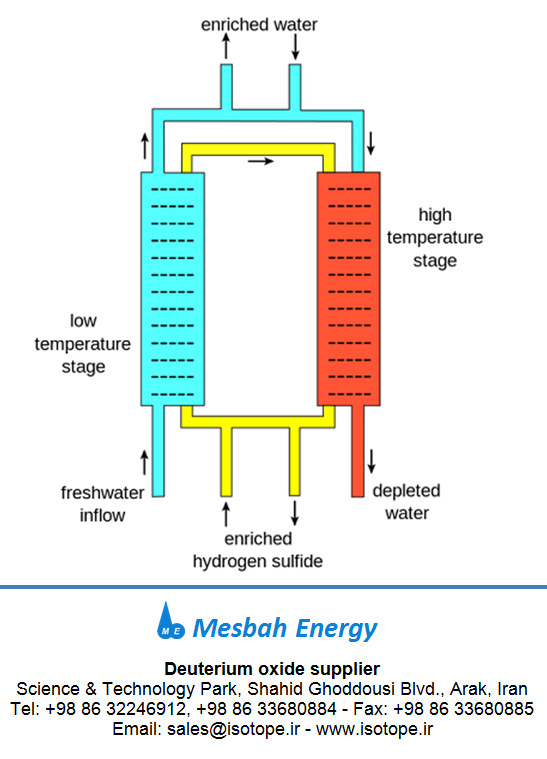 Fig 1.Production of deuterium oxide (heavy water) - Girdler sulfide
process
As semi heavy water, HDO occurs naturally on
Earth in regular water at a proportion of 1 part per 3200, it may be separated
from regular water by distillation or electrolysis and also by various chemical
exchange processes, all of which exploit a kinetic isotope effect. In short,
the difference in mass between the two hydrogen isotopes translates into a
difference in the zero-point energy and thus into a slight difference in the
speed at which the reaction proceeds. Once HDO becomes a significant fraction
of the water, deuterium oxide (heavy water) will become more prevalent as well
as water molecules trade hydrogen atoms very frequently. To produce pure
deuterium oxide by distillation or electrolysis requires a large cascade of
stills or electrolysis chambers, and consumes large amounts of power, so the
chemical methods are generally preferred. The most important chemical method is
the Girdler Sulfide process.
Source:
https://en.wikipedia.org/wiki/Heavy_water#Production History of deuterium oxide...
Fig 1.Production of deuterium oxide (heavy water) - Girdler sulfide
process
As semi heavy water, HDO occurs naturally on
Earth in regular water at a proportion of 1 part per 3200, it may be separated
from regular water by distillation or electrolysis and also by various chemical
exchange processes, all of which exploit a kinetic isotope effect. In short,
the difference in mass between the two hydrogen isotopes translates into a
difference in the zero-point energy and thus into a slight difference in the
speed at which the reaction proceeds. Once HDO becomes a significant fraction
of the water, deuterium oxide (heavy water) will become more prevalent as well
as water molecules trade hydrogen atoms very frequently. To produce pure
deuterium oxide by distillation or electrolysis requires a large cascade of
stills or electrolysis chambers, and consumes large amounts of power, so the
chemical methods are generally preferred. The most important chemical method is
the Girdler Sulfide process.
Source:
https://en.wikipedia.org/wiki/Heavy_water#Production History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 220
 Fig 1.Deuterium
oxide can be used to manufacture the Fiber Optics
The distinctive
behavior arising out of the isotope & deuterium bonding effects have found
potential use in various industrial scopes. Various applications have been
developed in the high tech area using some of the fundamental differences
between hydrogen and deuterium.
Optical fibers are widely
used when transmitting data over longer distances and at higher bandwidths than
traditional copper cables. Replacing the hydrogen with deuterium oxide in the fiber
cables reduces the chemical reaction rate leading to deterioration of the light
transmission, improves the intensity and transmission characteristics and extends
the life of the cable. History of deuterium oxide...
Fig 1.Deuterium
oxide can be used to manufacture the Fiber Optics
The distinctive
behavior arising out of the isotope & deuterium bonding effects have found
potential use in various industrial scopes. Various applications have been
developed in the high tech area using some of the fundamental differences
between hydrogen and deuterium.
Optical fibers are widely
used when transmitting data over longer distances and at higher bandwidths than
traditional copper cables. Replacing the hydrogen with deuterium oxide in the fiber
cables reduces the chemical reaction rate leading to deterioration of the light
transmission, improves the intensity and transmission characteristics and extends
the life of the cable. History of deuterium oxide...برچسب : نویسنده : 4deuteriumoxide3 بازدید : 147
